The laboratory operates under supervision of the Slovak National Accreditation Services (No. G-027) in accordance with the principles of Good Laboratory Practice (GLP). It focuses on developing experimental vaccines for zoonotic bacterial infections and non-clinical testing of antigens for in vitro diagnostic purposes. It includes Biosafety Level 3 (BSL3) facility.
Experimental Vaccines
- Against coxiellosis (Q fever) for small ruminants
Diagnostic antigens
- Coxiella burnetii Phase I and II: White to pale-yellow suspension of highly purified, killed cells in phosphate buffer with thimerosal; used for CFT. IFA or ELISA assays for Q fever diagnosis.
- Chlamydial infections: White to pale-yellow suspension of highly purified, killed cells of Chlamydia abortus or Chlamydia psittiaci in phosphate buffer with thimerosal; suitable for CFT, IFA or ELISA assays for psittacosis and enzootic abortion of ewes diagnosis.
- Rickettsial infections: White to pale-yellow suspension of highly purified, killed cells of Rickettsia rickettsii, Rickettsia akari, Rickettsia conorii, Rickettsia prowazekii, and Rickettsia typhi in phosphate buffer with thimerosal; suitable for CFT, IFA or ELISA assays for rickettsialpox, epidemic typhus, and murine typhus diagnosis.
Sera
Hyperimmune rabbit serum containing specific antibodies against C. burnetii, C. psittaci, C. abortus, R. akari, R. prowazekii, and R. typhi. Preservative: sodium azide
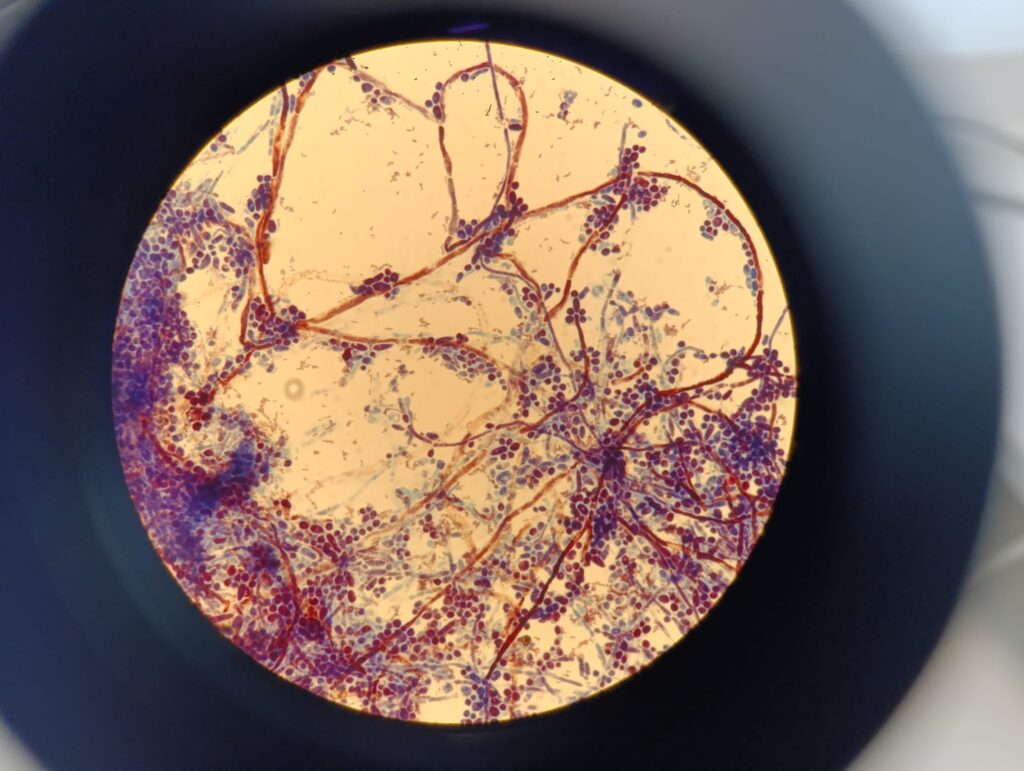
Photo Gallery


Contact



